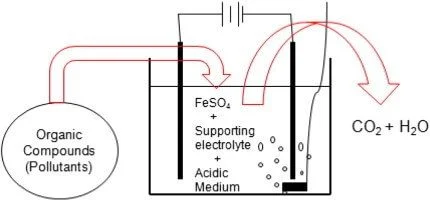
The Fenton Process for Wastewater Treatment
Fenton process has been an effective technique for the treatment of wastewater comprising of toxic refractory and organic pollutants. Fenton process comprise of advanced oxidation processes (AOPs) developed for the improvement of biodegradability of organic pollutants through oxidation process. Fenton process is more popular as compared to other AOPs due to its wider range of application, rapid degradability and mineralization, simpler operation and strong ability for anti-interference.
The Fenton Process, a potent oxidative method for wastewater treatment, stands out for its effectiveness in pollutant degradation, while a comprehensive discussion on Packaged Type Water Treatment Systems highlights their versatile applications. The paradigm shift towards No Sludge Treatment signifies an eco-friendly approach, minimizing environmental impact in modern water treatment practices.
Process
H.J.H Fenton discovered the Fenton reaction in 1894 and reported that H2O2 could be activated by ferrous (Fe2+) salts to oxidize tartaric acid. Eq. (1) is recognized as Fenton reaction and implies the oxidation of ferrous to ferric ions to decompose H2O2 into hydroxyl radicals. It is usually considered as the core of the Fenton chemistry. Furthermore, other reactions must be considered to understand the whole process:
Fe2+ + H2O2 →Fe3+ + OH– +OH, k2:1 =40-80 L mol-1 s-1
Operation parameters such as concentration of Fenton process, pH of wastewater, concentration of Fenton reagent, and initial organic pollutants concentration, of which wastewater pH are important for deciding the degradation efficiency of organic pollutants in the Fenton process.