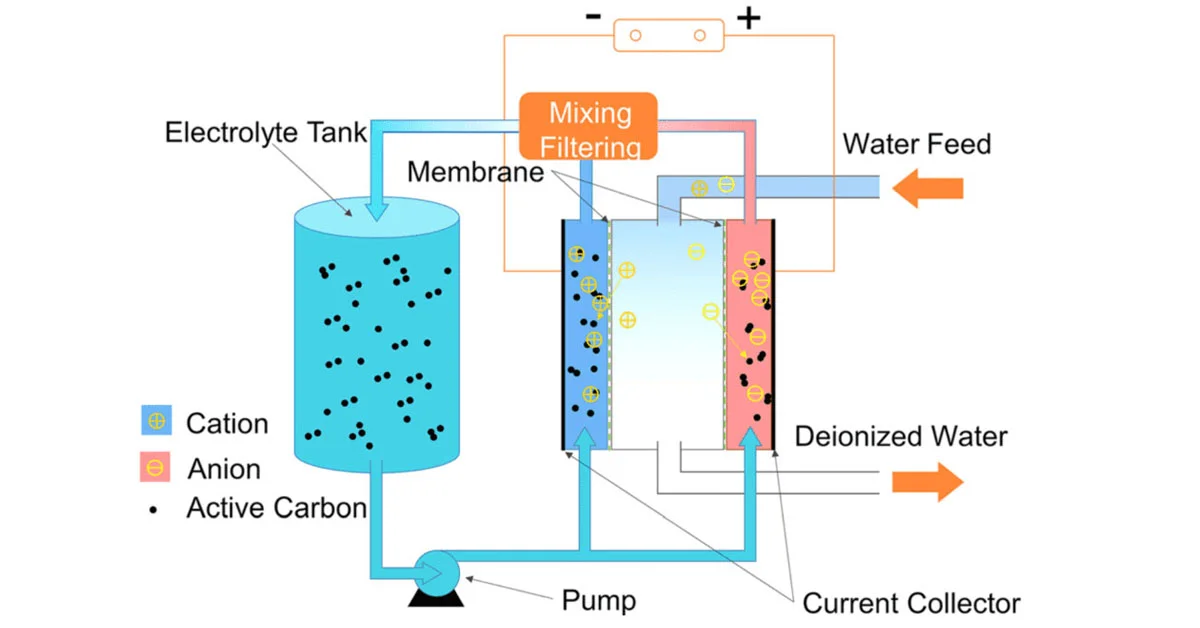
Capacitive deionization CDI is an electro-sorption technique that employs a sorption medium and an electrical field to separate ions and charged particles. In this process, positively polarized electrodes remove and retain anions from the water, while cations are similarly held within the negatively polarized cathode. CDI operates by creating an electrical potential difference between two electrodes, usually made of porous carbon.
capacitive deionization CDI is now mostly used to desalinate brackish water with a salinity of less than 10 g/L. Distillation, reverse osmosis, and electrodialysis are just a few of the processes used to deionize water. CDI is a more energy-efficient brackish water desalination process than reverse osmosis and distillation. This is due to the fact that CDI extracts salts from the feed water, whilst other technologies do extract water from the feed water.
Adsorption phase for desalinating water
A desorption phase for regenerating the electrodes comprises the two stages of operation in a traditional capacitive deionization CDI system. A potential difference between the two electrodes is applied during the adsorption phase, and ions are adsorbed from the water. The ions are transferred from the interparticle pores to the intraparticle pores in CDI with mostly porous carbon electrodes, where they are electrosorbed in the electrical double layers (EDLs).
The adsorbed ions are released for electrode renewal once the electrodes have been saturated with ions. The voltage difference between the electrodes is lowered or reversed. As a result, ions exit the electrode pores and can be flushed out of the CDI cell, yielding a high-salt brine or concentrated effluent stream. During the desorption step, some of the energy input required during the adsorption phase can be recover
Benefits of Using capacitive deionization CDI
1. Scalable and simple to operate
capacitive deionization CDI, unlike membrane or thermal processes, does not require high pressures or temperatures, making it an effective as well as affordable technology for salts removal.
2. Low energy cost for treatment of brackish water
The energy cost per volume of treated water scales closely with the amount of removed salt in capacitive deionization CDI, whereas it scales roughly with the volume of treated water in other methods like reverse osmosis. As a result, CDI can be used to desalinate low-salt streams like brackish water.
3. Solar Power Integration
capacitive deionization CDI Can work with solar power, still many works need to be done.
Working of capacitive deionization CDI cell
CDI cells have the capability to operate in two modes: either with a constant voltage or a constant current. The efficiency of a CDI cell relies on the choice of premium electrode materials. Carbon is the most commonly used porous material for electrodes. When considering the structure of carbon materials, several factors come into play. Achieving a high salt electrosorption capacity requires that the carbon interacting with ions possesses a significant specific surface area and a well-distributed pore size.
Furthermore, the material must be stable, with no chemical breakdown (degradation) of the electrode taking place within the CDI voltage window. When comparing CDI to reverse osmosis for water with salt concentrations less than 20 m M, a lab-scale study reveals that MCDI’s energy utilization in kWh per m3 freshwater generated is lower

Implementation over Large Scale
In 2007, ESTPURE enhanced the quality of reclaimed water by establishing a full-scale CDI facility in China, capable of processing 10,000 tonnes per day. This resulted in a reduction of total dissolved solids (TDS) from 1,000 to 250 mg/L and a decrease in turbidity from 10 to 1 NTU. Water recovery rates of up to 75% are attainable through this process. Unlike reverse osmosis, where there is some rejection, CDI eliminates rejection since it extracts ions from the water.